New paper in PTRSB: “Three-dimensional visualization of the cardiac ryanodine receptor clusters and the molecular-scale fraying of dyads”
Monday 03 October 2022
Our latest paper has been published in a cardiac special issue in Philosophical Transactions of the Royal Society B.
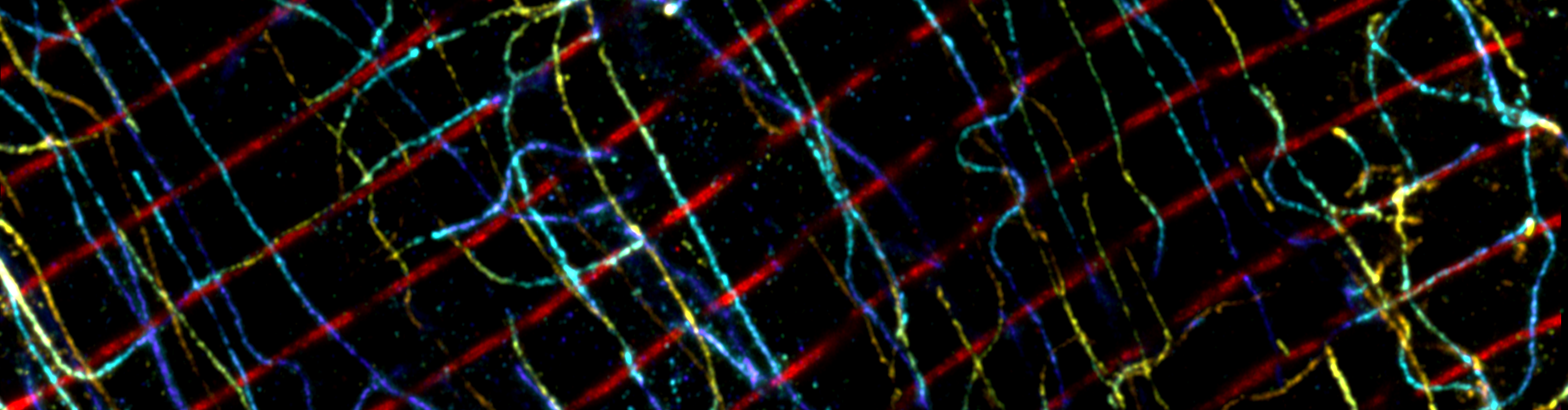
In this paper, we use expansion microscopy (ExM) to visualise the 3D geometry of dyadic calcium signalling nanodomains (clusters of calcium channels called ryanodine receptor 2 (RyR)) in heart cells. We also investigate the organisation of the anchoring protein junctophilin-2 (JPH) in dyads and observe how the JPH structural subdomains are remodelled in the pathological scenario of right ventricular heart failure.
We capitalised on the molecular-scale capabilities of enhanced expansion microscopy (X10 ExM with Airyscan), including the ~35 nm axial resolution and ability to image deep into a sample and in 3D, to fully appreciate the organisation and geometry of calcium-signalling nanodomains, by the use of immunofluorescent labelling of the RyR calcium channels.
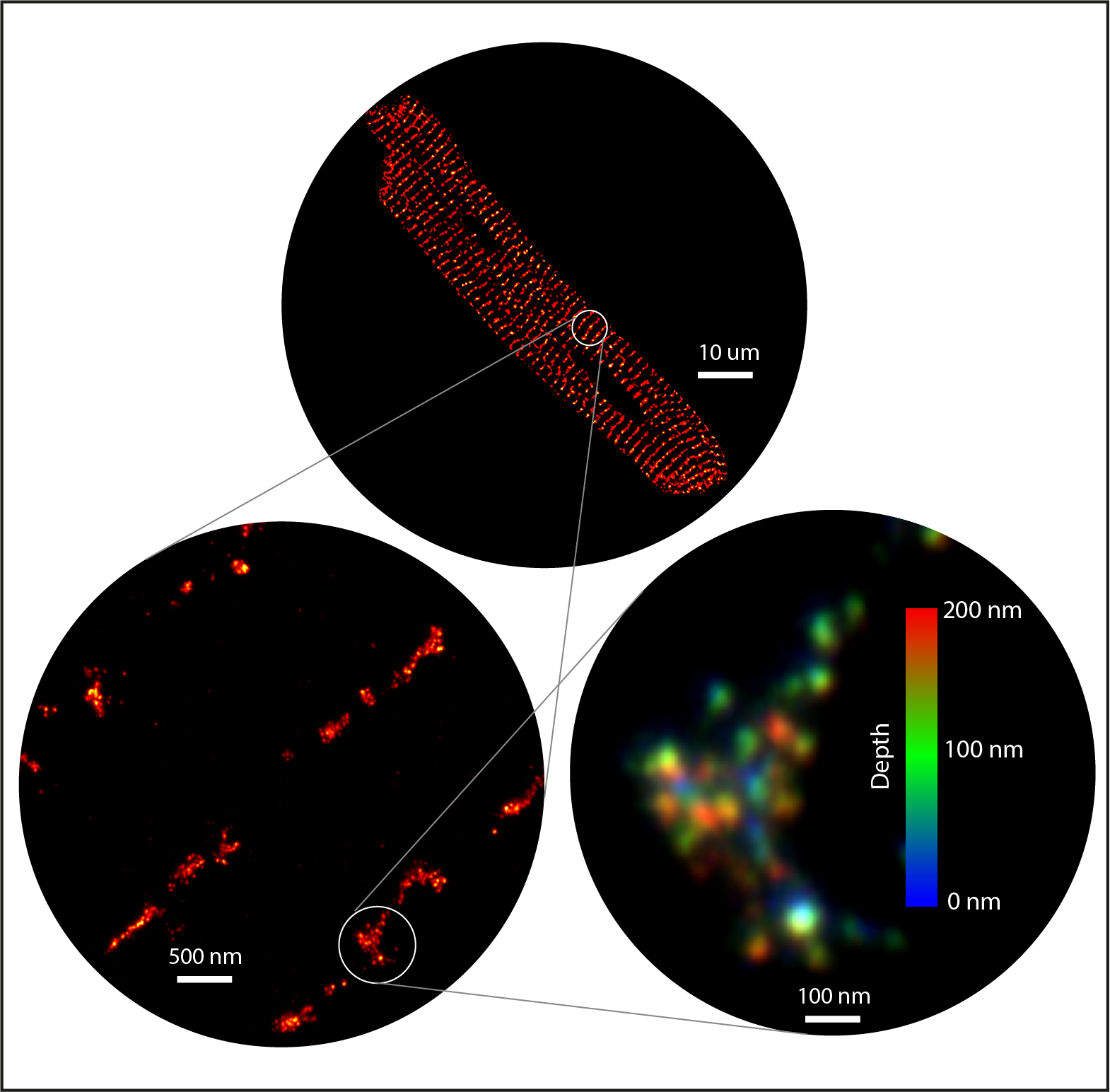
The 3D geometry of dyadic nanodomains, including fine cluster substructure, could be revealed only when the highest imaging resolution was obtained with the 10x variant of ExM (red).
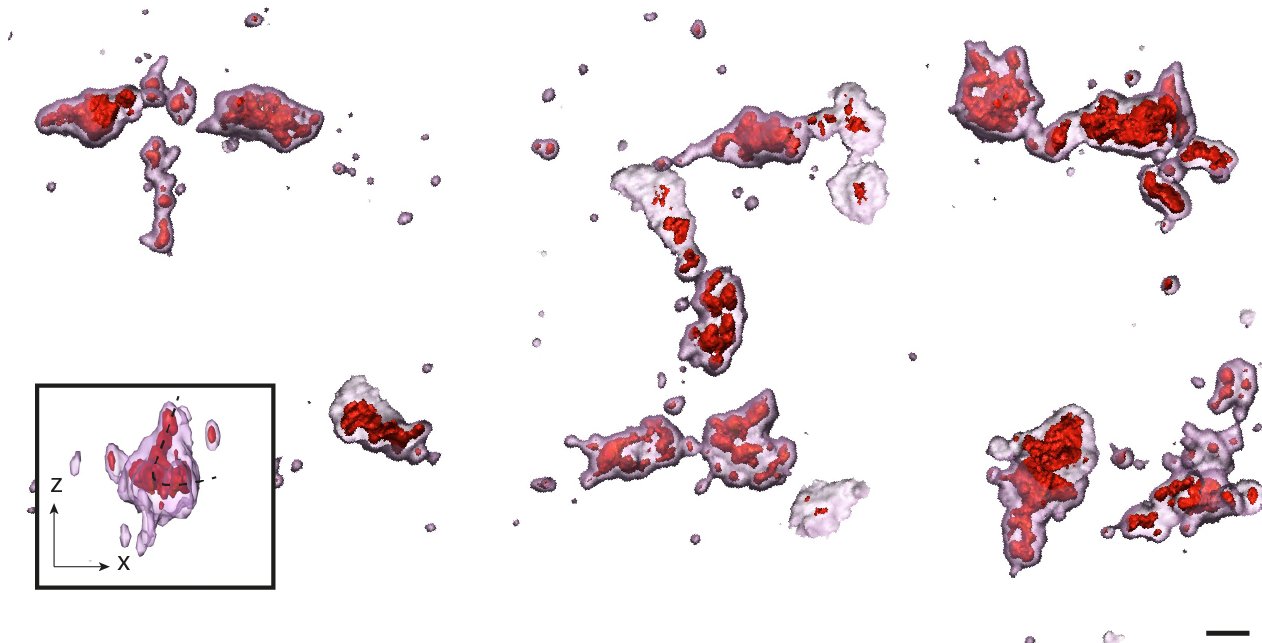
We explored how the dyadic architecture related to the adjacent t-tubule network by making use of immunocytochemistry and immunohistochemistry approaches. We observed several categories of dyad shape, which relate to the differing orientations and geometries of the t-tubules.
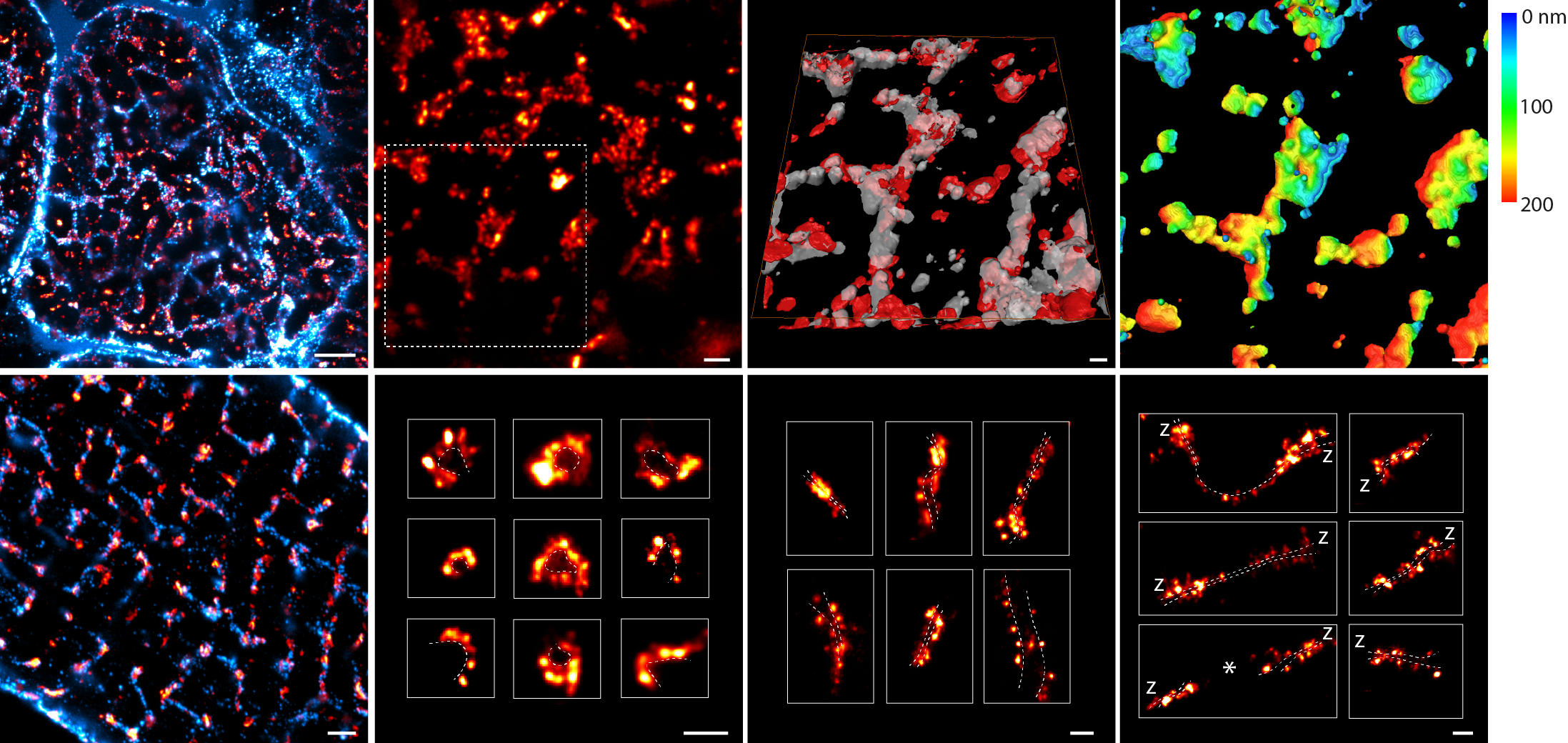
Using 2-colour imaging we observed how JPH anchors are organised alongside RyR. JPH commonly occupied a central sub-domain in the dyad, which may confer greater structural stability. The poles of the dyad typically lacked JPH, and here the RyR were organised more broadly. These poles with accessible RyR proteins may represent turnover domains.
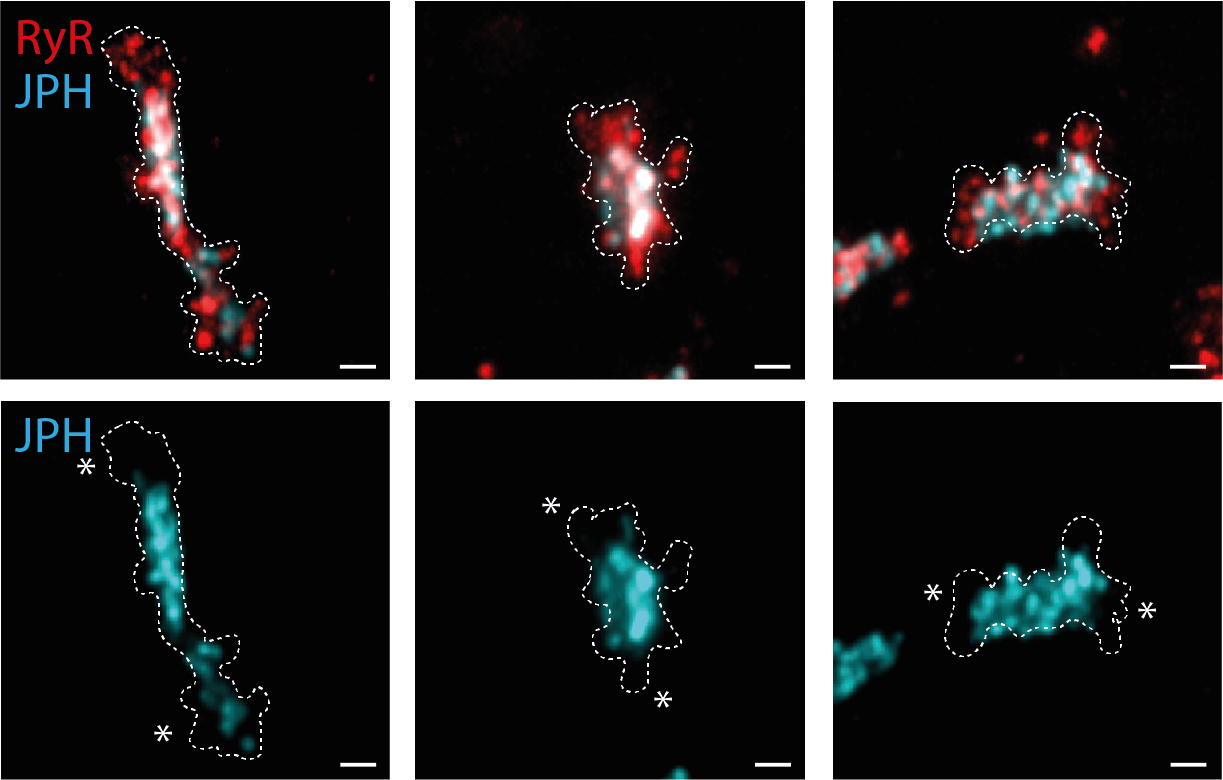
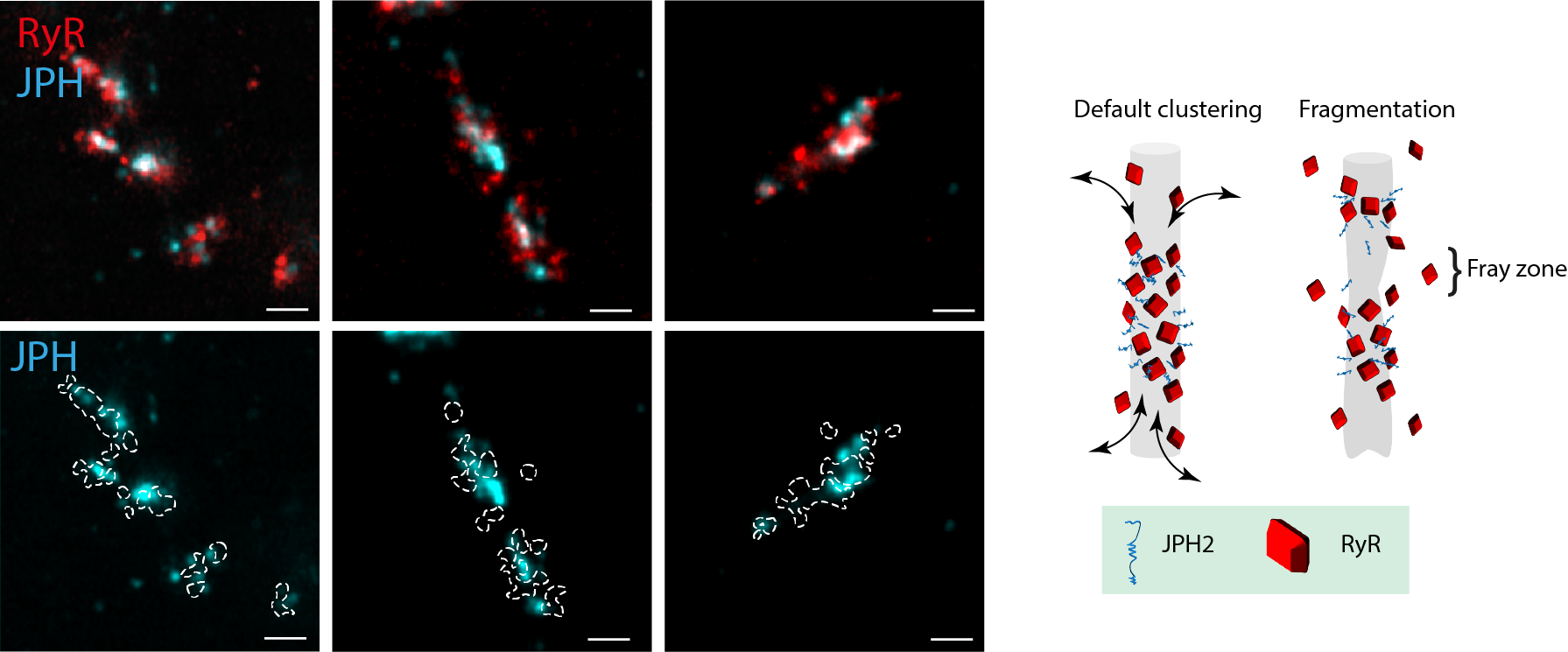
Finally, we observed remodelling of the dyadic JPH subdomains in monocrotaline-induced heart failure, entailing fragmented organisation, linked to fewer JPH and a fraying of the RyR morphology. The destabilisation of RyR arrays inside the JPH subdomains could be a critical stage in the dyadic remodelling that exists in heart failure.
Access the paper in the special issue in Philosophical Transactions of the Royal Society B here.