Pre-print online: RyR peptides as super-resolution imaging probes
Monday 29 June 2020
Our pre-print is now online! We used peptide ligands of the cardiac ryanodine receptor (RyR) as super-resolution imaging probes.
The research was conducted on placement with biotechnology company Badrilla.
The peptides used were the scorpion venom toxin imperacalcin, and CPVT-mimicking DPc10. We evaluated the specificity of the fluorescent peptide conjugates for the target RyR2 in fluorescent imaging experiments, in terms of colocalisation with RyR2 immunolabelling, with confocal and expansion microscopy.
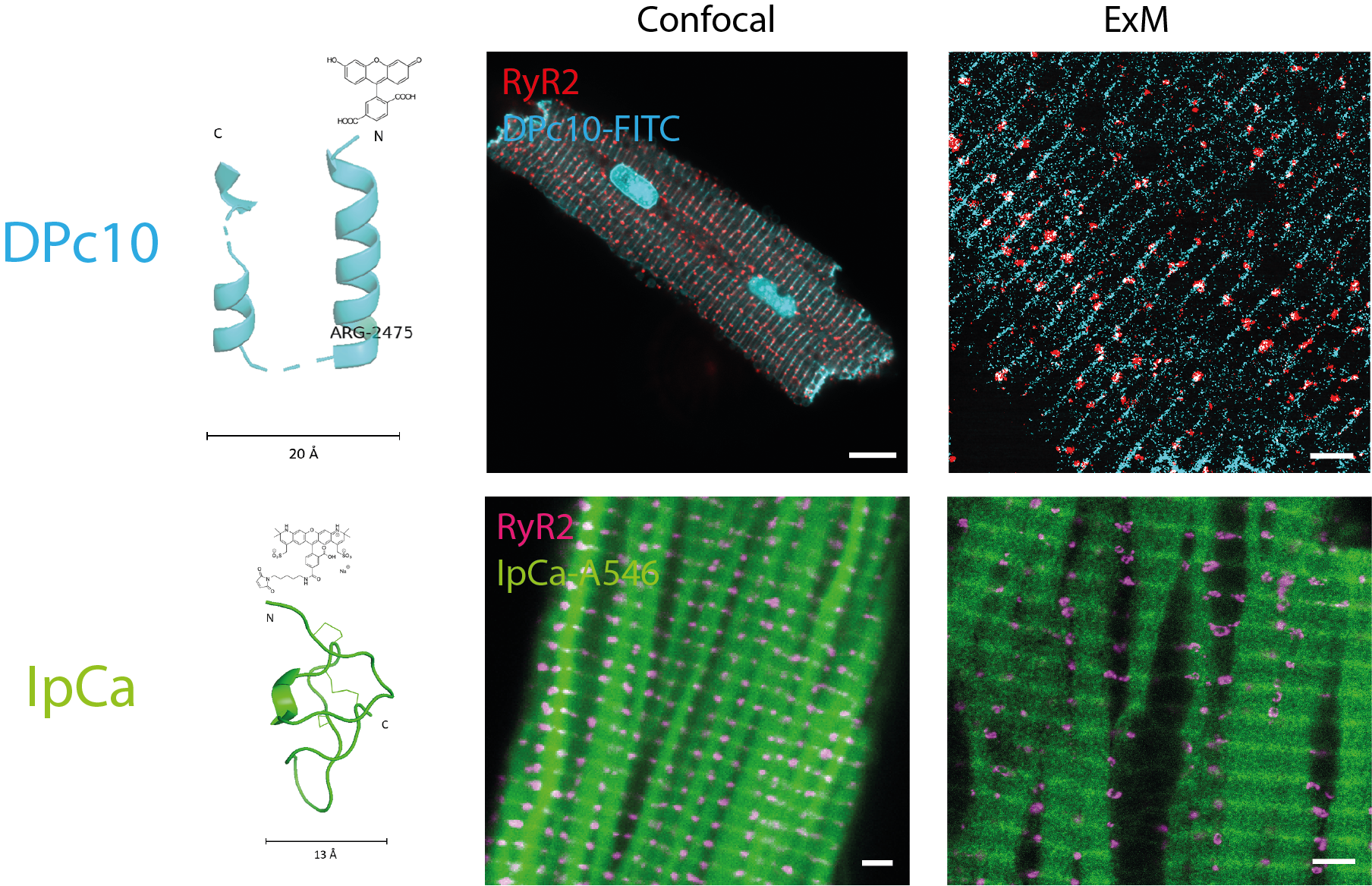
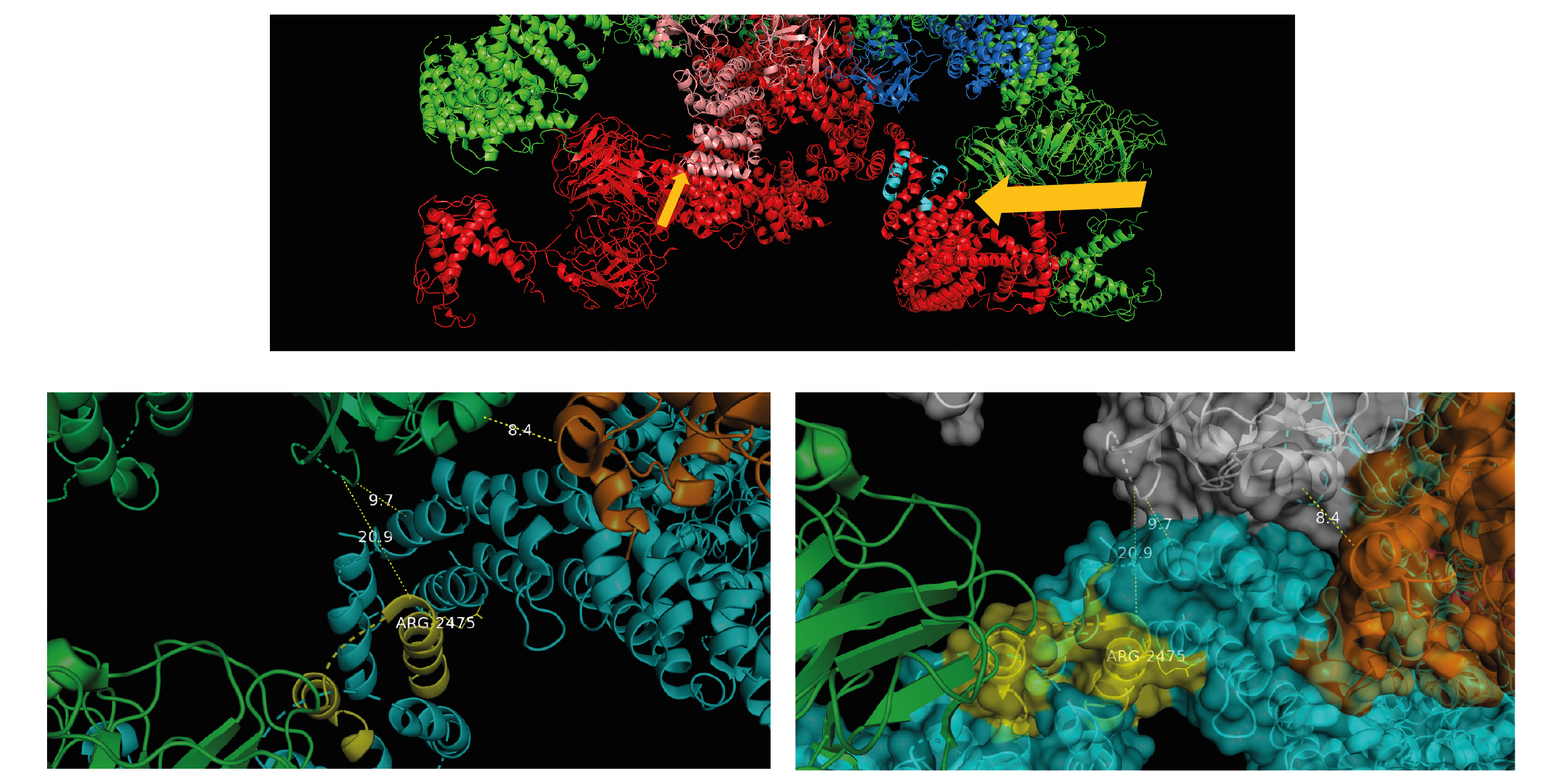
We also performed structural work looking at the DPc10 binding site on RyR2 atomic models to learn more about the peptide's mechanism of disturbance (peptide binding to RyR2 causes domain unzipping and destabilises the channel).
Thanks to the DiMeN DTP for funding the industrial placement, the host company Badrilla, and the other authors for help with the research! Research was undertaken during my mid-PhD year placement with Badrilla, experimental work cut short due to covid-19. Feedback on all contents of the work is welcomed.
Access the pre-print in bioRxiv at: https://www.biorxiv.org/content/10.1101/2020.06.26.131854v1.